Testing for Protozoa
 |
Test for
protozoa:
1. Label 15 Petri dishes, one for each protozoa sample taken in each plot. (ex: Plot 1A. Plot 1B. Plot 1C. etc.)
2. Place Plot 1 sample A into the bottom of its clean, corresponding petri dish; and allow to dry completely. Repeat in separate, corresponding petri dishes for all extractions.
3. Label another set of petri dishes.
4. Grind up dried Plot 1A with a mortar and pestle until almost completely powdery. Be sure to wash the mortar and pestle before using again. After grinding completely, store in a small plastic cup labeled with the sample name (ex. Plot 1A). Repeat for each sample into their corresponding, clean plastic cups.
|
|
5. Sift 9-10 g of Plot 1A into a 2nd clean petri dish using a 1 mm2 nylon screen or mesh into its corresponding labeled petri dish. Repeat for all samples into their corresponding, clean petri dishes.
|
|
6. Record the weight of each sample in grams.
7. Add 20 ml of distilled water to each sample to saturate the sifted soil.
8. Cover
each petri dish with its lid and allow to sit for at least 7 hours
12. Using a spatula, scrape the damp soil sample from the Plot 1A petri dish into its labeled, corresponding modified Uhlig extractor containing 30 mL of distilled water. Repeat for each sample, placing into the Uhlig extractor with the corresponding label. Let sit for 24 hours (see troubleshooting page for time management suggestions).
|
|
13. Remove the filtrate from Plot 1A and filter a 2nd time using 12.5 cm qualitative paper into a corresponding plastic cup. (seen below) Repeat for each sample into its corresponding plastic cup (see troubleshooting page for time management suggestions).
|
|
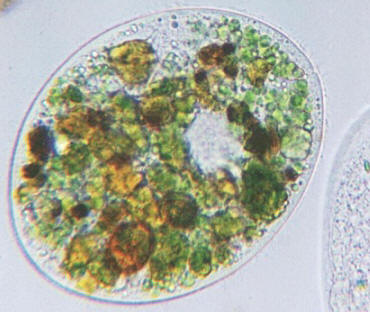
14. Deposit 7 μl of methyl-green stain on a clean microscope slide (see troubleshooting page). Then using a disposable graduated Beral-type pipette, add 18 μl (the first demarcation on the pipette) of the 2nd filtrate of Plot 1A from step 12 to the stain on the microscope slide and cover with an 18 x 18 mm2 cover slip.
15. Examine under a light microscope at 40X at five different views (see figure 1 for placement of the views). Count protozoa in each view. Add all protozoa together and divide by 5 for the average.
|
|
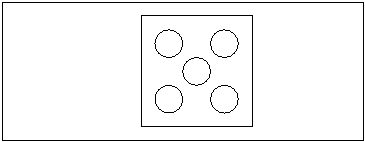 Figure 1 |
16. Repeat steps 13 and 14 for each sample. Record data in the corresponding area on a table.
17. Use the following equation to determine the population density of protozoa in the soil sample:
[(Average number of protozoa in fields)*(total ml of water used) *747] ∕ (grams of sifted soil) = # of protozoa per gram of soil.
18. Repeat this equation for all of the data from all of the plots.