Iron is considered 1 of 16 essential nutrients for
plants. It is needed in small amounts, and a lack of it can cause fatal
diseases in plants. Iron plays a monumental role in the production of
chlorophyll, which gives plants their green color. The diseases caused
by iron deficiency can be recognized when a plant's leaves turn a yellow
color. This color can slowly progress into black and the plant
could die if the problem is not addressed.

The amount of iron in soil can be affected by a number of conditions. The conditions examined in this experiment are those of compaction, saturation, and pH. The pH value of soil reveals a lot about the amount of nutrients available. This is because when soil has a lower or more acidic pH, more nutrients can be dissolved for plants to use. This causes a complimentary relationship between pH and many nutrients. When there is a high or alkaline pH, there is a lower amount of iron available in the soil, and when there is a low or acidic pH, there is a high amount of iron in the soil. Some plants thrive in acidic soil, but others prefer a more alkaline soil. Even though nutrients are more readily available in acidic soil, this can actually hurt plants more than help them because they can absorb nutrients in such high concentrations that it can be fatal. Usually, the best pH for soil is between 6-7.

Over the past 16 years, an irregularity in the amount of iron was observed consistantly in one site. It has the highest pH and contained the highest amount of iron. Our group decided to take on this unsolved mystery. After observing the site, it was hypothesized that the irregularity in iron was caused by the high saturation and compaction in the soil, along with the smaller plants' inability to absorb a lot of iron through the packed soil.
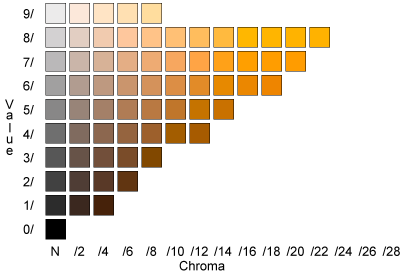
During our experiment, it was necessary to be able to uniformly identify the saturation and compaction of the soil. This was achieved by using a Munsell scale. The Munsell scale contains multiple shades of different colors that could be matched with the soil samples. By matching the correct colors, one is able to find out which soil samples are more or less saturated. If you are interested in learning more about the relationship between compaction and water levels in the soil on the amount of the iron in the soil, this experiment will lead you in the right direction!
For more information about the Munsell scale!
http://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/edu/?cid=nrcs142p2_054286