Experimenting:
Some steps for Bacteria and Protozoa are done at the same time. These are step 5 of Bacteria data collecting and step 1 of Protozoa data collecting.
Day One:
- Form three different 1 meter by 1 meter plots on a hill with a canopy that prevents rainwater from getting to the ground, causing dry soil. Choose a location with the same kind of plants throughout the space needed for all three plots.
Note:
Space the plots approximately 3 cm away from each other, but all next to
each other horizontally on the contour line (same elevation) of the hill. If placed above or below
another plot, runoff could transfer from one plot to another,
jeopardizing the experiment.
-
Take 9 soil samples 15 cm deep and 2 cm in diameter, 3 from each
plot, deposit each in a clean Ziploc bag, and label to allow samples
to be distinguished from each other.
-
Note:
To take a soil sample, use a soil core extractor to drill a hole 15 cm
into the ground. Twist clockwise until 15 cm into the ground, and then
carefully pull out the extractor and transfer the soil into a storage
vessel.
-
Space out the locations of the extractions as to get a more accurate
assessment of the plot’s protozoa and bacteria levels.
-
Place 15 cm sample of soil sample into the bottom of a clean, empty
petri dish; and allow to dry completely.
-
Wet plot 2 with 4 liters of distilled water by slowly pouring,
allowing the soil the chance to absorb the water before pouring
more. Attempt to limit runoff.
-
Wet plot 3 with 8 liters of distilled water by slowly pouring,
allowing the soil the chance to absorb the water before pouring
more. Attempt to limit runoff. Plot 1 is left as the negative
control.
Day Two:
6.
Repeat step 2 for the “wet” soil samples.
7.
Place 15 cm sample of soil sample into the bottom of a clean, empty
petri dish; and allow to dry completely. For us, this took upwards of 24
hours.
Data Collecting:
Bacteria:
Day Two:
1. Using a clean, new transfer pipette to add 10 ml distilled water to a
15 ml culture tube. Label the tube 100 and the name of the
sample being worked on (Ex: 1A, 1B, 1C, 2A, etc).
1.
Use the same pipette to add 9 ml to a second 15 ml culture tube. Label
the tube 10-1.
2.
Repeat step 2 three more times to three additional 15 ml culture tubes,
only label them 10-2, 10-3, and 10-4
respectively.
3.
Repeat steps 4-6 for the other eight samples.
4.
Label bacterial plates to correspond to all labelled culture tubes and
set aside.
Go here
to find out where to buy bacteria media growth plates
Serial Dilutions for Bacteria:
5.
Place 1 cc of your soil sample into the 100 culture tube
6.
Cap
the tube and shake vigorously
NOTE: In the following steps, only use a pipette ONCE
7.
Using a clean, new pipette, remove 1 ml of the soil/water mixture from
the 100 tube and place in the 10-1 tube
8.
Cap and shake vigorously
9.
Remove 1 ml of the soil/water mixture from the 10-1 tube and
place in the 10-2 tube
10.
Cap and shake vigorously
11.
Remove 1 ml of the soil/water mixture from the 10-2 tube and
place in the 10-3 tube
12.
Cap and shake vigorously
13.
Remove 1 ml of the soil/water mixture from the 10-3 tube and
place in the 10-4 tube
14.
You should now have a total of five culture tubes for one sample.
15.
Repeat steps 1-13 for the other eight samples.
16.
Re-shake the culture tubes.
17.
Plate 100 ul samples
18.
Allow to grow for 48 to 72 hours.
Day Four:
19.
Repeat steps 1 – 18 for the
“wet” samples.
For the “original” samples:
20.
Examine each of the plates for the individual bacteria colonies and
choose the plate with the fewest colonies (but at least 5). Start at the
most diluted plate and only analyze lower dilutions if there are not 5
visible red spots on the plate. To make your
estimates of the number of bacteria in the original 1 cc soil sample
using the following formula:
# Microbes in 1 cc of soil = # Colonies on sheet x 10-2 x 10|dilution
# at which these colonies were found|
Day Seven:
21.
Repeat step 20 for the “wet” samples.
Protozoa:
1.
In
the lab, sift 9-10 grams of soil into a 2nd clean petri dish using a 1
mm2 nylon screen or mesh. Place mesh in sterilizing solution
for at least two hours, and then clean.
Note: Use remaining sifted soil to begin steps 1-14 of Serial Dilutions for Bacteria
2. Add 20 mL of distilled water to saturate the soil.
3.
Cover the petri dish with its lid and allow to sit for 7 hours. Put
in refrigerator after 7 hours if you aren’t going to work on it
immediately.
4.
Place in a
modified Uhlig extractor containing 30 ml of distilled water for 24
hours
5.
Remove the filtrate and filter the “original” samples a 2nd time using
12.5 centimeters qualitative filter paper.
6.
Using a capillary tube, deposit 7 ul of methyl-green stain on a clean
microscope slide (1 ul= 1 drop from the capillary tube). Then using a
disposable graduated Beral-type pipette, add 18 ul (the first
demarcation on the pipette) of the 2nd filtrate from step 6 to the stain
on the microscope slide and cover with an 18x18 mm2
coverslip.
7.
Examine under a light microscope at 40x (for quantitative) or 100x (for
qualitative) observations of the various protozoa living in the soil.
8.
Use this equation to determine the population density of protozoa in the
soil sample: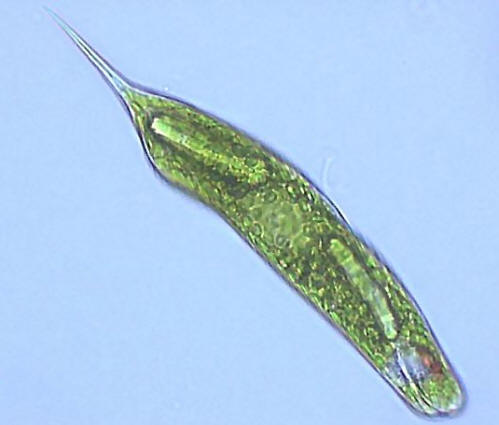
[(# per field of view at 40X) x (total ml of water used) x 747] / (grams of sifted soil) = # of protozoa per gram of soil.
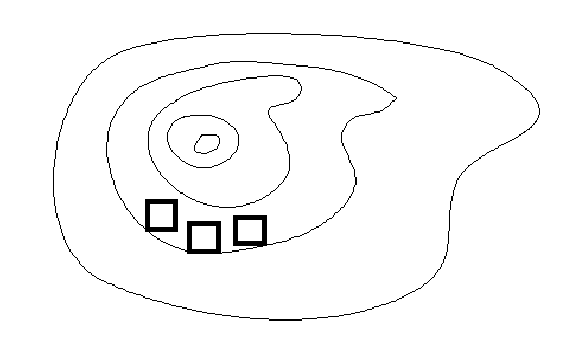
An example of plot placement along a countour line
Using soil core extractors to gather soil samples
Soil samples layed out to dry
Soil in the soil core extractor
Measuring distilled water for the serial dilutions
Labeling bacteria growth plates
Analyzing bacteria growth plates and counting bacteria!
Carefully recording all data and performing the correct calculations
Uhlig Extractors prepped and ready!
Uhlig Extraction process
Set up to filter the soil a second time!
Making the protozoa slides
Counting protozoa!
Lots of testate amoeba in this field of view